J-coupling is an interaction transmitted through electron clouds in which one nuclear spin perturbs the spin of another in the same molecule. Two types of J-coupling are seen in phosphorus spectroscopy: homonuclear (between two adjacent ³¹P nuclei) and heteronuclear (between nearby ³¹P and ¹H nuclei). As described in a prior Q&A, J-coupling produces spectral line-splitting. The magnitude of the splitting is given by the coupling constant (J) which is measured in Hz.
A good example of homonuclear J-coupling can be seen in the ATP molecule, where the coupling constant between adjacent ³¹P nuclei is a respectable 16 Hz. This results in a visually apparent splitting of the α- and γ-peaks into doublets by coupling with the ³¹P-nucleus at the β-position.
A good example of homonuclear J-coupling can be seen in the ATP molecule, where the coupling constant between adjacent ³¹P nuclei is a respectable 16 Hz. This results in a visually apparent splitting of the α- and γ-peaks into doublets by coupling with the ³¹P-nucleus at the β-position.
Heteronuclear coupling between ³¹P and ¹H is often more subtle and insidious as the JP-H coupling constants are relatively small (<3-5 Hz). Discrete line splitting is hard to observe and the coupling appears to broaden the ³¹P lines rather than cleave them neatly. Removing this coupling between ³¹P and ¹H (a process known as proton decoupling) will thereby improve the quality of the spectra as illustrated below.
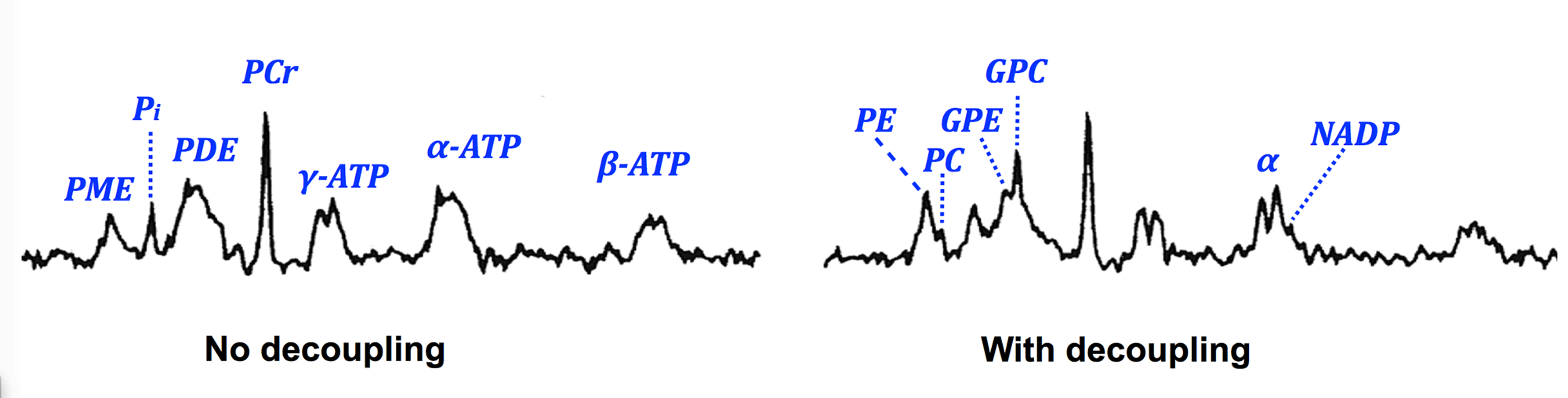
³¹P brain spectra with and without ¹H decoupling. "Membrane peaks" are especially affected: PME is increased in amplitude with improved resolution into Phosphoryethanolamine (PE) and phosphorylcholine (PC); PDE peak resolved into GPE (glycerophosphoethanolamine) and GPC (glycerophosphocholine); α-ATP doublet better resolved with NAD now seen on shoulder.
¹H-decoupling is performed by applying RF-irradiation at the ¹H Larmor frequency only during the period when the ³¹P signal is being recorded. The decoupling irradiation causes each ¹H nucleus to change its state rapidly and lose memory/reference to the state of its coupled ³¹P partner. The effect is instantaneous and disappears as soon as the decoupling irradiation is turned off. Two decoupling methods are widely available:
- Continuous wave decoupling. Narrow-bandwidth RF-irradiation centered near the ¹H Larmor frequency runs continuously during data acquisition. This must be carefully tuned and is sensitive to magnetic field inhomogeneities. RF-energy deposition in tissues may be limiting.
- Broadband decoupling. Instead of using continuous excitation, broadband techniques apply a series short composite pulses that are cleverly phase cycled to disrupt J-coupling. The WALTZ-4 method is currently the most popular.
As shown in the example above, proton decoupling is most useful for visualizing and characterizing PDE and PME resonances. If information about these peaks is of interest (as it usually is in brain, liver, kidney, and tumor spectroscopic evaluation), the proton decoupling is required. However, if one is only interested in following the major energy metabolites (such as PCr and ATP), as is often the case in MRS of the heart or skeletal muscle, then proton decoupling may not be beneficial.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Jung W-I, Staubert A, Widmaier S, et al. Phosphorus J-coupling constants of ATP in human brain. Magn Reson Med 1997; 37:802-4.
Li CW, Negendank WG, Murphy-Boesch J, et al. Molar quantitation of hepatic metabolites in vivo in proton-decoupled, nuclear Overhauser effect enhanced ³¹P NMR spectra localized by three-dimensional chemical shift imaging. NMR Biomed 1996; 9:141-155.
Luyten PR, Bruntink G, Sloff FM, et al. Broadband proton decoupling in human ³¹P NMR spectroscopy. NMR Biomed 1989; 1:177-183.
Shaka AJ, Keeler J, Freeman R. Evaluation of a new broadband decoupling sequence: WALTZ-16. J Magn Reson 1983; 53:313-340.
Jung W-I, Staubert A, Widmaier S, et al. Phosphorus J-coupling constants of ATP in human brain. Magn Reson Med 1997; 37:802-4.
Li CW, Negendank WG, Murphy-Boesch J, et al. Molar quantitation of hepatic metabolites in vivo in proton-decoupled, nuclear Overhauser effect enhanced ³¹P NMR spectra localized by three-dimensional chemical shift imaging. NMR Biomed 1996; 9:141-155.
Luyten PR, Bruntink G, Sloff FM, et al. Broadband proton decoupling in human ³¹P NMR spectroscopy. NMR Biomed 1989; 1:177-183.
Shaka AJ, Keeler J, Freeman R. Evaluation of a new broadband decoupling sequence: WALTZ-16. J Magn Reson 1983; 53:313-340.
Related Questions
Why do some spectra split into smaller peaks while others do not?
What must you do differently to perform ³¹P spectroscopy in lieu of ¹H spectroscopy?
What is NOE? How and when should it be used for phosphorus MRS?
Why do some spectra split into smaller peaks while others do not?
What must you do differently to perform ³¹P spectroscopy in lieu of ¹H spectroscopy?
What is NOE? How and when should it be used for phosphorus MRS?
